Authors: Fatema Jaffer, Riad Hosein / Editor: Lauren Fraser / Reviewer: Sandi Angus / Codes: ACCS LO 2, CC6, NeoC3, SLO5 / Published: 19/02/2024
Congenital heart disease is the most common of all congenital malformations, affecting nearly 10 in every 1000 newborns. [1]. Despite advances in prenatal and newborn screening, patients may still present undiagnosed to ED. This is because the heart is transitioning from the foetal to neonatal circulation.
Presentations range from life-threatening shock or cyanosis in a neonate, to respiratory distress, congestive cardiac failure (CCF) and failure-to-thrive in infants. As 80-85% of patients with CHD now survive to adulthood (Best et al, 2016), patients can also present later in life with complex needs.
Congenital Heart Disease describes any defects in the structure of the heart or great vessels that is present at birth or in early childhood. [3]
Incidence

Fig.1 The prevalence and percentages of 27 CHD subtypes1
Foetal Circulation
Foetal circulation relies on 3 shunts to direct blood away from the developing lungs and liver. The lungs are filled with fluid during development which causes a high resistance to pulmonary blood flow.
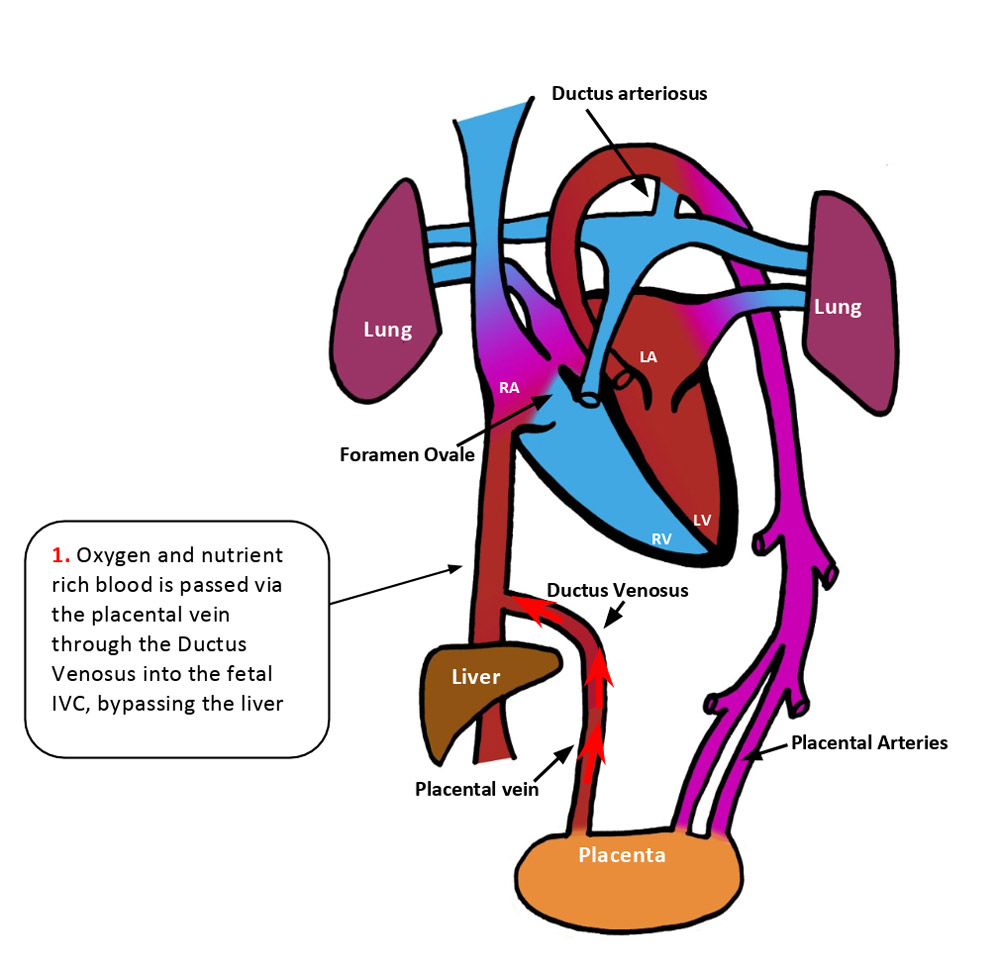




During gestation, the Foramen Ovale acts as a flap valve and is held open by the difference in pressure between the right and left atria. The left atrium has a low pressure because there is little blood returning from the lungs whereas pressure in the right atrium is higher as it receives systemic venous return.
Transition to Neonatal Circulation




Duct dependent lesions
- The timing of presentation of CHD can be related to whether lesions are duct-dependent or not. Some neonates with CHD remain dependent on the Ductus Arteriosus to allow mixing of blood to maintain systemic or pulmonary circulations.
- Presentation of these lesions is usually around the time the DA closes, which occurs due to withdrawal of prostaglandin E2. In the foetus, prostaglandin E2 production is very high because of the placenta and this plays a role in keeping the Ductus Arteriosus open. At birth, prostaglandin levels fall due to proper functioning of the lungs, changes in neonatal blood pressures in the systemic and pulmonary circulations and removal of the placenta.
- They can either present be with subtle symptoms when the duct is just beginning to close such as poor feeding, sleepiness and tachypnoea
- Or can present as the collapsed neonate with cyanosis and shock when closure has occurred whereby a Prostaglandin infusion is required to maintain patency.
- For some duct dependent lesions, a Blalock-Taussig (BT) shunt is used to maintain blood flow to the pulmonary artery
- A synthetic graft is used to create a shunt between the right subclavian artery and the right pulmonary artery
- Another surgical shunting method is to create a conduit between the right ventricle and the pulmonary artery (RV-PA Conduit)
Learning Bite
Foetal circulation relies on three shunts which close shortly after birth. Some CHD lesions depend on DA patency so present with cyanosis/shock when this closes. These are the duct-dependent lesions.
Presentations of CHD
In order to characterise congenital heart abnormalities, it is useful to split them into how they may present to the ED. The most common presentations of CHD in the neonatal and early childhood period are shock, cyanosis or congestive heart failure. It is important to note that these present differently in children compared to adults.

Table showing timing of presentation of CHD (Judge et al, 2016). Abbreviations TAPVD: Total Anomalous Pulmonary Venous Drainage, TOF: Tetralogy of Fallot, TGA:Transposition of the Great Arteries, HLHS: Hypoplastic Left Heart Syndrome, AS: Aortic Stenosis, PDA: Patent Ductus Arteriosus, ASD: Atrial Septal Defect, VSD: Ventricular Septal Defect.
Shock results from inadequate oxygen delivery to meet metabolic demands of the tissues. When CHD presents as shock, it is often due to symptomatic obstruction of the left ventricular outflow tract. Adequate blood flow depends on a patent ductus arteriosus therefore when this closes after birth, systemic blood flow decreases.
Causes of Neonatal shock/collapse
Learning Bite
The five main causes of circulatory collapse in the neonate are:
- Sepsis
- CHD
- Metabolic/Endocrine disturbances (including inborn errors of metabolism)
- NAI
- Trauma
Another useful way to remember common causes of neonatal collapse is the mnemonic “THE MISFITS”.
| T | Trauma |
| H | Heart disease |
| E | Endocrine |
| M | Metabolic/electrolyte disturbance |
| I | Inborn errors of metabolism |
| S | Seizures/CNS abnormalities |
| F | Formula errors e.g over-dilution |
| I | Intestinal disorders e.g Intussusception |
| T | Toxins |
| S | Sepsis |

Learning bite
Apnoea is a common side effect of Prostaglandin infusions – ensure senior help is available when considering an infusion. It is important to know where Dinoprostone is kept in your department. It may also be useful to run/attend simulation scenarios around shock in the neonate.
- Co-arctation of the aorta (severe)





Co-arctation is not always severe. Lesions are defined as critical when the systemic circulation depends on ductus arteriosus patency. It can also remain asymptomatic until adulthood. It is often associated with other abnormalities such as a VSD or bicuspid aortic valve.
Examination/investigation findings
- Examination:
- Absent femoral pulses or Radio-femoral delay before the DA closes,
- Cyanosis
- 4 limb BP discrepancies – gradient of >10mmHg between upper and lower limbs is clinically significant
- Signs of CCF
- Investigation
- Diagnosis is usually made antenatally via USS scan
- ECG may show LVH or RV conduction delay
- CXR: may show figure of 3 sign (see below)
- All patients should have either cardiac MRI or CT for evaluation of thoracic aorta prior to definitive management

Management
- Rapid A-E assessment of collapsed neonate
- Prostaglandin infusion if duct-dependent circulation
- Diuretics to reduce preload combined with volume replacement to correct metabolic acidosis
- Surgery usually performed within 24 hours – removal of the narrow segment
- Hypoplastic left heart syndrome




This is a common diagnosis with around 260 cases in the UK annually (Barron et al, 2009). It is usually diagnosed prenatally. Initially, most patients with HLHS will be haemodynamically stable. Over several days, as pulmonary vascular resistance falls and the DA closes, the ratio of pulmonary to systemic blood flow will increase. This causes increased right→left shunting which leads to cyanosis, congestive heart failure, shock and respiratory distress.
Examination/Investigation findings
- Examination:
- Absent femoral and brachial pulses, delayed CRT, hypotension secondary to shock
- Signs of right heart failure – enlarged liver, tachypnoea, rales
- Shock and respiratory distress secondary to acidosis upon DA closure
- May have PDA murmur (see section on PDA)
- Investigations
- ECG: absent LV forces
- CXR: cardiomegaly
- Echo is gold standard for diagnosis
Management
- Patients should be born in a cardiac centre
- Prostaglandin infusion to maintain DA patency
- Surgery within 3-5 days – surgical management is staged and requires 3 operations to establish a single ventricle circulation (Hosein et al, 2007)
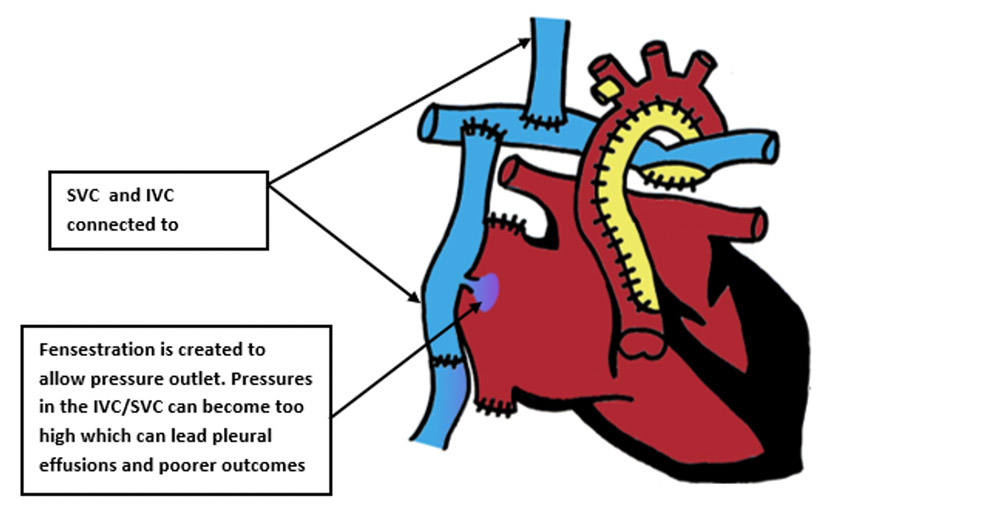
Norwood Procedure → neonatal period – creation of common atrium by removal of atrial septum, Pulmonary arteries attached to a reconstructed aortic arch to create a single outlet right sided heart. Pulmonary circulation provided by BT shunt (shown) or an RV to PA conduit.

- Glenn → 4-6 months – SVC connected to pulmonary arteries. Venous return from the head and neck is routed directly to the lungs which allow saturations to reach 80-85%. The BT shunt/conduit is removed. By 3-5 years, there will be insufficient blood returning from the head and neck to keep the child well so a Fontan is required.

- Fontan → 3-5 years – IVC connected to the pulmonary circulation so venous return from the body is routed directly to the lungs which allows saturations to reach 90-95%. Most patients with a Fontan circulation reach adulthood but must undergo long term follow up. Many are able to manage school/jobs and some female patients are even able to undergo pregnancy and delivery!
Learning bite
The most common CHD lesions that can present as shock are severe co-arctation of the aorta and hypoplastic left heart syndrome. Both require surgical correction.
Cyanosis is defined as a bluish discolouration of the skin and mucous membranes resulting from an inadequate amount of oxygen in the blood. Cyanosis in infants can be central or peripheral. In some cases, peripheral cyanosis can be normal e.g following cold exposure. Central cyanosis however is always pathological. In the context of CHD, cyanosis is caused by right→left shunting. This causes de-oxygenated blood to mix with oxygenated blood which is then pumped around the body. Cyanotic babies may present collapsed, with blue discoloration of the hands, feet and tongue. It is important to examine carefully as cyanosis can be missed in children with dark skin and is also harder to detect in patients who are anaemic.
Learning bite
5 main CHD lesions causing Cyanosis
- 1: Truncus Arteriosus – 1 common artery
- 2: Transposition of the Great arteries – 2 switched arteries
- 3. Tricuspid Atresia – 3 leaflets of the tricuspid valve are undeveloped
- 4. Tetralogy of Fallot – 4 defects with one cause
- 5. TAPVD – 5 words make up the lesion name
Differentials to consider for the cyanosed baby


Learning bite
Hyperoxia test is used to distinguish between cardiac and respiratory causes of cyanosis in neonates.
- Truncus arteriosus




Examination/Investigations
- Examination
- Wide pulse pressure
- Systolic ejection murmur – increased flow across semilunar valve
- Single second heart sound
- Investigation
Management → Surgical repair via a Rastelli procedure. This involves a patch to close the VSD and allow the LV to communicate with the common trunk. A Conduit is then made from the RV to the pulmonary artery.
- Transposition of the great arteries
This occurs due to failure of septation of the pulmonary artery and aorta.



Investigations
- ECG – usually normal
- CXR: may show egg on string appearance or signs of CCF
Management
- Where there is no VSD, when the Ductus Arteriosus closes → cyanosis → ABCDE, prostaglandin infusion
- If there is a VSD, patients are usually well oxygenated however they are more prone to developing CCF so require surgical repair within the first few weeks
- Some infants with TGA and an intact ventricular septum will remain excessively cyanotic despite initiation of PGE infusion and these patients require a balloon atrial septostomy to create an ASD
The definitive management is an arterial switch operation. This is often carried out in the first few weeks to avoid left ventricular deconditioning.
Learning bite
Without a shunt (VSD/ASD/PDA), Transposition of the Great Arteries is incompatible with life as there is no entry for oxygenated blood to the systemic circulation
- Tricuspid atresia


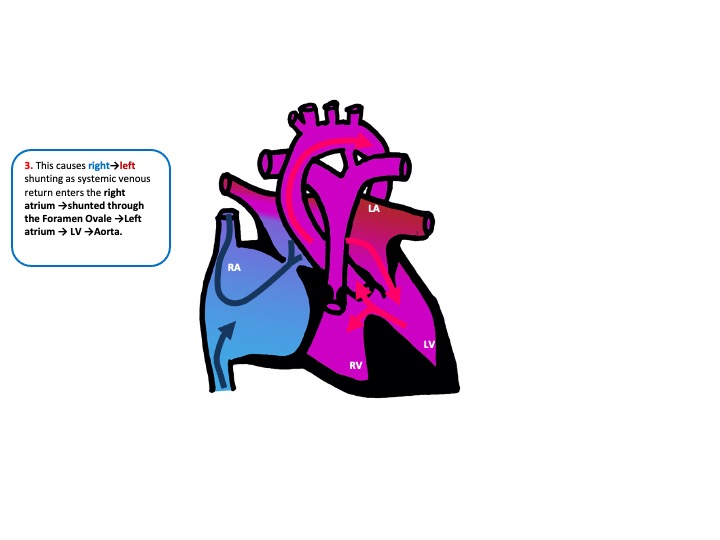
Examination and Investigation findings
- Examination: Harsh systolic murmur
- ECG: superior axis, absent RV voltages, large P-waves
- CXR: May have increased or decreased pulmonary vascular markings
Management
- If the lesion presents with cyanosis →BT shunt or RV to Pulmonary artery conduit shortly after birth
- If it presents with CCF, medical management is used initially followed by surgical correction with a Pulmonary Artery band to reduce pulmonary vascular resistance and pressure.
- Definitive management is to establish Fontan circulation by 3-5 years of age
Learning bite
Truncus arteriosus and Tricuspid atresia are both lesions that can present with cyanosis at birth and a harsh systolic murmur due to flow through a semilunar valve/VSD
- Tetralogy of Fallot


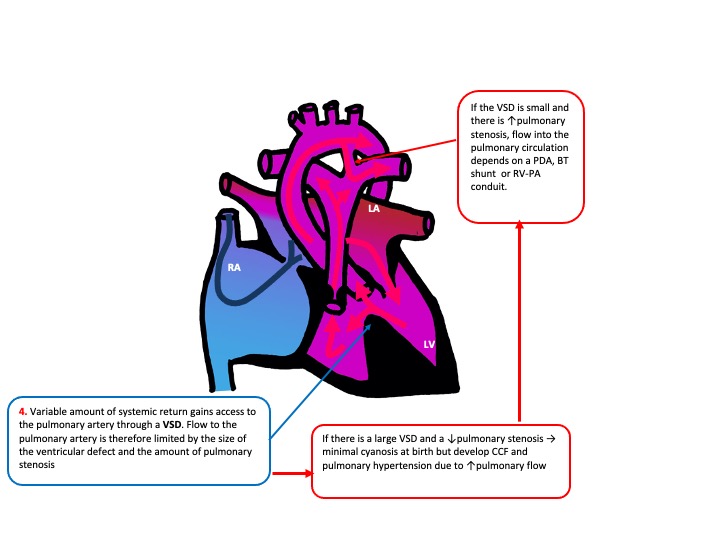
TOF presentation depends on degree of RV outflow obstruction as this determines the extent of mixing of oxygenated and deoxygenated blood.
- If there is a large degree of pulmonary stenosis the entire right ventricle output passes through the VSD. Pulmonary flow is only supplied by the DA → presents with cyanosis when the DA closes
- If there less pulmonary stenosis, pulmonary blood flow will be higher → CCF and cyanosis later in life (hypercyanotic “tet spells”)
Pathophysiology and Management of “Tet Spells”
- This occurs when there is hypoxemia of sudden onset E.g following systemic vasodilation after a meal or warm bath
- Hypoxemia → ↓in systemic vascular resistance →↓afterload→ ↑right to left shunting →worsens hypoxemia →metabolic acidosis → respiratory distress → May progress to unconsciousness /convulsions due to acidosis
Below is a short video detailing the pathophysiology and management of acute hypercyanotic spells
Investigations
- ECG: RA and RV enlargement
- CXR: boot shaped heart due to RA and RV enlargement resulting in an upturned apex with an absent or diminished pulmonary artery
- Echo/cardiac MRI +/- cardiac catheterisation – gold standard for diagnosis
Management
- 10% require BT shunt or RV to Pulmonary Artery conduit in newborn if severely cyanosed
- Acute cyanosis at birth is managed with Prostaglandins
- Most have elective surgical repair at 6-9 months
- This involves using a patch to close the VSD and surgical widening of the stenosed pulmonary valve.
Following surgery 85% progress to adulthood
Learning bite
Management of hypercyanotic “tet spells” involves:
- Placing the child in a knee to chest position
- Administration of high flow oxygen
- Morphine
- Get senior help – the child is likely to require an admission to PICU
- Total anomalous pulmonary venous drainage
When this occurs, the four pulmonary veins do not drain into the left atrium but drain into the innominate vein(Supracardiac), liver (Infracardiac ) or coronary sinus/right atrium (cardiac).




Presentation
- Depends on the degree of obstruction between pulmonary veins and right heart
- In Supracardiac and Infracardiac lesions, there is usually a higher degree of obstruction which presents at birth with pulmonary oedema, pulmonary hypertension and cyanosis →collapsed cyanotic neonate
- In cardiac lesions there is less obstruction → Develop symptoms within weeks to months – mild cyanosis and CCF
Investigation findings
- ECG: Right axis deviation, RVH
- CXR: snowman sign
- Definitive imaging: Echo, cardiac MRI, angiography
Management
- If patients present obstructed – emergency surgical correction
- If non obstructed – medical management of CCF (see section on the breathless baby) followed by elective surgical repair
Learning bite
In Supracardiac and Infracardiac lesions, the pulmonary veins must pass through accessory vessels and structures such as the diaphragm and liver therefore there is a higher degree of obstruction to flow. These lesions therefore present earlier.
Congestive heart failure – the breathless baby
- Heart failure is a syndrome caused when the heart is unable to supply blood to meet tissue demands. In the context of CHD, heart failure is caused by shunting of blood from left→ right.
- As oxygenated blood has a higher pressure, when it is shunted to the right side of the heart, there is increased flow through the pulmonary vasculature.
- This congestion results in pulmonary oedema and breathlessness.
- As the total volume being pumped into the systemic circulation is lower, this also results in poor tissue perfusion which causes fatigue, poor nutrition and failure to thrive.
Learning bite
Congestive cardiac failure as a result of CHD often presents in the first 1-3 months of life. This is because pulmonary blood flow is initially restricted by high pulmonary vascular resistance which starts to decline after birth which leads to increased shunting.
Clinical features of CHD in newborns/infants
The presentation of CCF in neonates is extremely different to the presentation in adults. Symptoms and signs of reduced cardiac output and reduced tissue perfusion may often be non-specific.
| Presentation | Symptoms | Signs | Potential Cardiac Lesions |
| CCF (Breathless baby) |
Feeding difficulty Sweating/tachypnoea with feeds Failure to thrive Respiratory distress Fussiness |
Tachypnoea and laboured breathing Rales Hepatomegaly Cyanosis if severe Faltering growth Displaced apex beat Cool peripheries Gallop rhythm |
VSD ASD PDA if large |
- Ventricular Septal Defect (VSD)

Examination/Investigation findings
- Examination:
- They are usually asymptomatic and undetectable unless large.
- Large lesions are defined as being the same size or larger than the aortic valve and larger lesions are often associated with pulmonary hypertension and present with CCF after one week
- May have pansystolic murmur at lower left sternal edge – usually the louder the murmur the smaller the hole
- Investigations:
- ECG: Biventricular hypertrophy by 2 months
- CXR: shows increased pulmonary vascular markings and cardiomegaly
Management
- Trial of medical management
- Loop diuretics such as Furosemide, Digoxin, ACE-inhibitors, Beta-blockers and Spironolactone are used.
- Supplemental oxygen and hyperventilation should be avoided as they will cause pulmonary vascular resistance to fall which causes increased shunting.
- Muscular VSDs often close spontaneously whereas perimembranous VSDs do not.
- These are closed either surgically or via percutaneous closure by an interventional cardiologist.
- Surgical correction is deferred to 3-5 months of age, as this has been shown to improve outcome.
- Atrial Septal Defect (ASD)


Examination/investigation findings
- Examination: Soft systolic murmur at upper sternal edge
- ECG: Partial RBBB, RV hypertrophy
- CXR: Cardiomegaly, increased pulmonary vascular markings
Management
- Medical management is used to treat symptoms of CCF.
- Surgical closure usually occurs at 3-5 years of age
- 90% undergo device closure in catheter lab , 10% undergo surgical closure if defects are large
Learning bite
ASDs and VSDs are managed medically to allow surgical closure to be deferred as this improves outcomes.
- Patent Ductus Arteriosus (PDA)
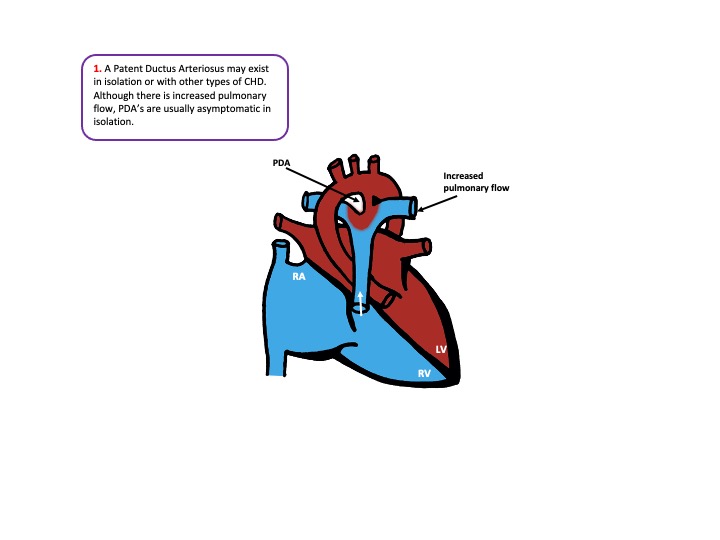

Examination/investigation
- Examination: Continuous systolic murmur in the left infraclavicular area – known as a machinery murmur
- Investigations
- ECG: Usually normal
- CXR: Increased pulmonary vascular markings if large
Management
- 2/3rds PDA’s will close with a course of indomethacin or Ibuprofen as these are prostaglandin inhibitors
- If large enough to cause CCF → medical management is used and surgical ligation is carried out at 1-3 months
- If there is no CCF → closure in catheter lab with a coil or device is carried out at 1 year
- There is a slightly increased risk of bacterial endocarditis if left patent
Learning bite
PDAs rarely cause symptoms in children – they are often picked up incidentally from the murmur
- Liu Y, Chen S, Zühlke L, et al. Global birth prevalence of congenital heart defects 1970-2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. 2019 Apr 1;48(2):455-463. doi: 10.1093/ije/dyz009.
- Somerville J. MANAGEMENT OF ADULTS WITH CONGENITAL HEART DISEASE: An Increasing Problem. Annual Review of Medicine, 1997. 48(1), pp.283-293.
- Judge P, Meckler Mshs G. Congenital Heart Disease In Pediatric Patients: Recognizing The Undiagnosed And Managing Complications In The Emergency Department. Pediatr Emerg Med Pract. 2016 May;13(5):1-28; quiz 27-8.
- Bernier PL, Stefanescu A, Samoukovic G, Tchervenkov CI. The challenge of congenital heart disease worldwide: epidemiologic and demographic facts. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2010;13(1):26-34.
- Erikssen G, Liestøl K, Seem E, Birkeland S, Saatvedt KJ, Hoel TN, Døhlen G, Skulstad H, Svennevig JL, Thaulow E, Lindberg HL. Achievements in congenital heart defect surgery: a prospective, 40-year study of 7038 patients. Circulation. 2015 Jan 27;131(4):337-46; discussion 346.
- MacDonald MG, Mullett MD, Seshia MMK, editors. Avery’s neonatology: Pathophysiology and management of the newborn. 6th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2005.
- Kouchoukos NT, Blackstone EH, Hanley FL. Kirklin/Barratt-Boyes Cardiac Surgery [Internet]. Elsevier Health Sciences; 2012.
- Beattie, R. and Champion, M., 2002. Essential Revision Notes In Paediatrics For The MRCPH. Knutsford, Cheshire: PasTest Ltd.
- Radiopaedia.org. 2020. Radiopaedia.Org, The Wiki-Based Collaborative Radiology Resource. [online] Available at: <https://radiopaedia.org/> [Accessed 12 June 2020].
- Kliegman, R., Stanton, B., St. Geme, J., Schor, N., Behrman, R. and Nelson, W., 2016. Nelson Textbook Of Pediatrics. 20th ed. Philadelphia: Elsevier.
- Brawn, W., Barron, D. and Jones, T., 2011. Hypoplastic left heart. Paediatrics and Child Health, 21(1), pp.19-24.
- Barron, D., Kilby, M., Davies, B., Wright, J., Jones, T. and Brawn, W., 2009. Hypoplastic left heart syndrome. The Lancet, 374(9689), pp.551-564.
- Hosein RB, Clarke AJ, McGuirk SP, Griselli M, Stumper O, De Giovanni JV, Barron DJ, Brawn WJ. Factors influencing early and late outcome following the Fontan procedure in the current era. The ‘Two Commandments’? Eur J Cardiothorac Surg. 2007 Mar;31(3):344-52; discussion 353.
- Best KE, Rankin J. Long-Term Survival of Individuals Born With Congenital Heart Disease: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 2016;5(6):e002846. Published 2016 Jun 16. doi:10.1161/JAHA.115.002846.




5 Comments
Excellent summary
thank you
I thought this was excellent and probably the best I have understood all this!
excellent summary of CHD. Really like the acute management strategies from ED perspective.
Excellent review