Author: Rosie Darwood / Editor: Jason Kendall / Reviewer: Jon Bailey, Peter Lynas / Codes: SLO1, TC1, VC1 / Published: 14/01/2022
Context and Definition
Acute limb ischaemia is defined as any sudden decrease in limb perfusion causing a potential threat to limb viability [1]. By convention this usually refers to patients presenting with symptoms for less than 2 weeks. The spectrum of acute limb ischaemia therefore ranges from the patient with a few hours history of a painful cold white leg, to the patient with a few days history of short distance claudication or the patient with a sudden increase in ischaemic symptoms on a background of peripheral arterial disease.
The incidence of acute limb ischaemia is estimated at approximately 1 per 6000 population per year [1], which means a hospital serving a population of 500,000 would expect to see 83 cases per year. Cohort studies from Sweden suggest the incidence is reducing [2]. A prospective population based study in Oxfordshire running from 2002-2012 determined the incidence and outcome of all acute peripheral arterial events in a population of 92728, finding 510 acute events in 386 patients requiring 803 interventions [3]. Risk factors include hypertension, smoking, and diabetes mellitus interval.
There is also some evidence that the proportion of acute limb ischaemia caused by embolic disease is falling, due to the decreased incidence of rheumatic heart disease and the improvement in the management of atrial fibrillation [1]. In this context, and with an ageing population, the presentation of acute on chronic limb ischaemia is more common. This can be more difficult to diagnose since the classical signs of acute limb ischaemia may be attenuated by the presence of collaterals. Even patients presenting primarily with embolic disease may well also have underlying peripheral arterial disease, which is present in up to 30% of people over the age of 70 years [4,5].
Learning Bite
Most acute limb ischaemia now occurs on a background of peripheral arterial disease.
Mortality and Morbidity
Acute limb ischaemia is important to diagnose because it carries a high mortality and morbidity.
Estimates of mortality following acute limb ischaemia range from 9-22% (1;6-8), but the timeframe of this mortality is important. In the Oxford study, of 386 patients 221 (59.3%) were 75 years of age, and 98 (26.3%) were 85 years old; 230 (62.3%) were independent before the event, but 270 (73.4%) were dead or dependent at 6-month follow-up, and 328 (88.9%) were dead or dependent at 5 years. The 30-day survival with acute limb ischemia was 75.3% compared with critical limb ischemia at 92.6%.
Limb salvage following acute limb ischaemia is estimated at 70-90% (1;7-10). Amputations are more common following thrombotic occlusions, since these are more likely to occur on a background of peripheral arterial disease(10) and therefore are more difficult to treat.
Learning Bite
Acute limb ischaemia carries a high morbidity and mortality.
Pathophysiology
An embolus is defined as a material (gas, solid or liquid) that is carried within the circulation and lodges in a blood vessel in another part of the circulation, causing occlusion of the blood vessel. Radiologically the upper border of an embolus is classically concave, known as the meniscus sign (See Figure 1). An embolus is distinct from a thrombus, which is a blood clot formed in situ within the vascular system of the body and impeding blood flow.
In acute limb ischaemia emboli most commonly arise from the heart (80%) and as such are usually composed of platelets. In the Oxford study, 23.6% of events were cardioembolic; of this group 70.5% had known atrial fibrillation diagnosed before the event. Emboli can also arise from proximal arterial disease (either aneurysms or stenoses) and may then contain atheroma. These carry a poorer prognosis for the limb since they are harder to treat and not amenable to thrombolysis.
Thrombosis may be influenced by any of the three factors described in Virchows Triad:
- Phenomena of interruputed blood flow, eg stasis: risks include venous stasis, long surgical operations, prolonged immobility, and varicose veins.
- Phenomena associated with irritation of the vessel and its vicinity, eg endothelial or vessel wall injury: includes injury or trauma such as vessel piercings, damage arising from shear stress or hypertension, and subsequent contact with procoagulant surfaces, such as bacteria, shards of foreign materials, biomaterials of implants or medical devices, membranes of activated platelets, and membranes of monocytes in chronic inflammation.
- Phenomena of blood coagulation, eg hypercoagulability: risk factors such as hyperviscosity, coagulation factor V Leiden mutation, coagulation factor II G2021A mutation, deficiency of antithrombin III, protein C or S deficiency, nephrotic syndrome, changes after severe trauma or burn, cancer, late pregnancy and delivery, race, advanced age, cigarette smoking, hormonal contraceptives, and obesity.
Rarer causes of acute limb ischaemia are shown in Table 1:
| Cause | Pathology | Signs to look for |
| Vasculitis | Inflammation of the arteries | Bilateral disease Systemic symptoms (e.g. fever, malaise)Other signs connective tissue disease |
| Popliteal entrapment syndrome | The popliteal artery is compressed by gastrocnemius during plantar flexion | Young man, sporty Pain brought on by exercise |
| Compartment syndrome | Swelling of tissues within fascial compartment (especially anterior compartment of leg) compresses artery | Look for history of trauma Pain on passive movement |
| Iatrogenic | Injury of usually the common femoral or superficial femoral artery following catheterization | History of catheterization (e.g. coronary angiogram) |
| Aortic dissection | The dissection flap may occlude the true lumen of a branch vessel causing end organ ischaemia | Back pain Hypotension Changing signs (e.g. pulses palpable) over time |
| Graft occlusion | Thrombosis of graft especially if prosthetic rather than vein | History of previous vascular surgery Look for scars Severity of ischaemia will depend on how quickly it has blocked (previous stenosis may allow collaterals to develop) |
The Classic presentation the 6Ps:
Classically a patient with acute limb ischaemia presents with the 6 Ps (see below) however these may be attenuated if there is a background of chronic ischaemia due to the presence of collaterals.
Pain In most cases this will occur at rest, although a patient with a viable limb may present with acute onset short distance claudication. Rest pain is usually worse in the most distal part of the limb (toes) since this has the worst perfusion, and may be relieved on dependency (hanging legs over bed). Pain which is worse on passive movement of the muscles indicates potential compartment syndrome (see below) and is a poor prognostic sign.
Pallor this is especially useful in comparison to the opposite limb; it is also useful to check venous filling. Acutely ischaemic limbs are classically white rather than blue. Chronic critically ischaemic limbs may appear pink due to compensatory vasodilation the so-called sunset foot . In this situation Buergers test may also be useful (pallor on elevation of the limb, with erythema on dependency).
Paraesthesia this is present in over 50% cases (1). Sensory nerves are smaller than motor nerves and more sensitive to ischaemia so tend to be affected first.
Paralysis this is a poor prognostic sign and indicates an element of irreversible ischaemia.
Perishingly cold this is a useful sign if used in comparison to the opposite (normal ) limb. Check temperature using the back of your hand.
Pulselessness checking pulses is notoriously unreliable. Arterial Doppler signals should be checked in anyone with suspected acute limb ischaemia (1). Audible arterial Doppler signals do not eliminate the diagnosis of acute limb ischaemia (see below).
Learning Bite
Classical signs (6 Ps) may be attenuated in a patient with pre-existing peripheral arterial disease and collaterals. Arterial Dopplers should be performed on all patients with suspected acute limb ischaemia.
Cardiovascular examination
A full cardiovascular examination should be performed, to detect cardiac arrhythmias or possible valvular heart disease as a source of emboli. The abdomen should be assessed for evidence of an abdominal aortic aneurysm.
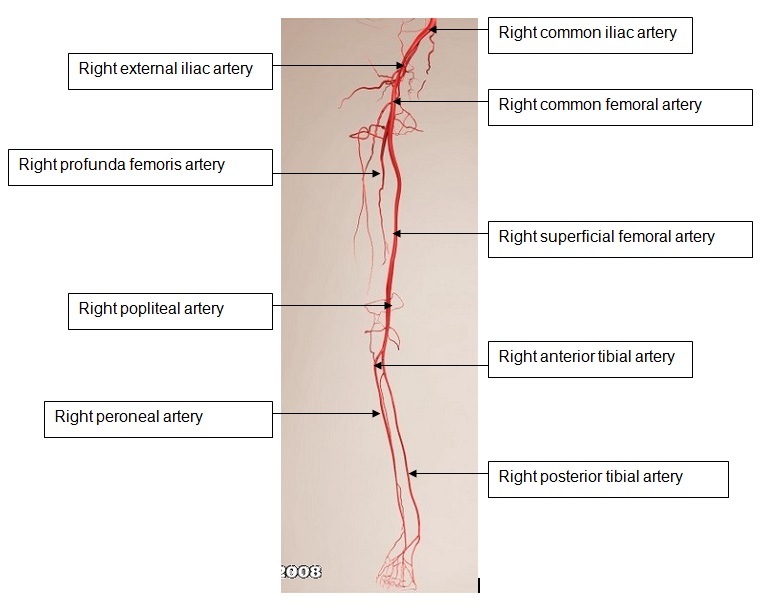
Figure 2 demonstrates the arterial anatomy of the lower limb.
Figure 2: The arterial tree of the lower limb
The affected leg
(i) Inspection
Colour: Look at the colour of the leg white suggests acute ischaemia. Fixed mottling of the leg is a poor prognostic sign and implies irreversible ischaemia. Chronically ischaemic legs may appear pink or blue. Dry gangrene (black tissue) is also a late sign and consistent with chronic irreversible ischaemia (more than 2 weeks).
Patients with classical emboli have a white leg (with a normal leg on the opposite side); in patients with thrombotic occlusions the signs may be more subtle since collaterals may have formed due to pre-existing peripheral arterial disease.
Scars: Look for scars of previous surgery. Surgery on the abdominal aorta may be via a midline or transverse incision, patients who have had an EVAR (endovascular abdominal aneurysm repair) will only have scars on the groin. Dont forget behind the knee patients who have had a popliteal aneurysm repair may have a vertical scar behind the knee.
(ii) Palpation
Temperature: Always compare to the opposite leg. It may also be helpful to assess the temperature of other peripheries (hands) and check the core temperature.
Pulses: It is particularly important to determine whether the patient has a palpable femoral pulse.
Tenderness: Is the limb tender? This again is a poor prognostic sign as it suggests muscle ischaemia. Is there pain on passive movement? This suggests compartment syndrome and requires immediate vascular referral for urgent intervention.
Neurological function: Test sensory and motor function. Loss of sensation is common. Loss of motor function is a poor prognostic sign. Any neurological deficit implies the need for emergency intervention.
(iii) Auscultation
Arterial Doppler signals: A Doppler examination should be performed on all patients with suspected acute limb ischaemia. Once again it is very important to compare these to the opposite leg. The ankle-brachial pressure index (ABPI) can be measured to help assess the severity of ischaemia when Doppler signals are audible. This is the systolic pressure in the pedal arteries divided by the brachial artery pressure.
A manual sphygmomanometer cuff is placed around lower leg and inflated until there is no audible pedal Doppler signal. The cuff is then slowly deflated until the arterial Doppler signal is audible this is the ankle pressure. The brachial pressure must be measured in the same way. Normal ABPIs range from 1.0-1.2. Most claudicants will have an ABPI of 0.6-0.8 and patients with critical ischaemia (defined as rest pain or tissue loss) usually have an ABPI of 0.2-0.4. Diabetics may have falsely elevated ABPIs due to calcified incompressible arteries.
The Other Leg
It is essential to fully examine both legs, the comparison between the normal and abnormal leg will often aid both diagnosis and determining probable aetiology.
Learning Bite
When examining always compare with the contra-lateral leg.
Classification
The purpose of the history and examination is to determine three things:
- Is the leg acutely ischaemic? (Or is there an alternative diagnosis?)
- Is the likely cause embolic or thrombotic?
- Is the leg viable, threatened or irreversibly ischaemic?
The answers to these three questions will determine the immediate management. It is not always possible to distinguish between embolic and thrombotic aetiology, since many patients with an embolic cause may also have some underlying peripheral arterial disease by virtue of their age (15-20% of patients >70 years old have peripheral arterial disease[4]). It is most important to decide whether the leg is viable, threatened or irreversibly ischaemic (See Table 2).
Table 2. Rutherfords classification of acute limb ischaemia
| Capillary return | Motor | Sensory | Arterial Doppler signal | Venous Doppler signal | |
| I Viable | |||||
| IIa Threatened (salvageable if promptly treated) | Intact/slow | Partial (toes only) or none |
x (often) |
||
| IIb Threatened (salvageable with immediate reconstruction) | Slow/absent | Partial paralysis | Partial (more than toes) or complete |
x (usually) |
|
| III Irreversible (major tissue loss or permanent nerve damage inevitable) | Absent+ staining |
x profound paralysis(rigor) |
x profound (anaesthetic) |
x | x |
Learning Bite
Sensorimotor deficit helps identify limbs in need of urgent intervention. Fixed staining and profound paralysis are signs of irreversible ischaemia.
Compartment Syndrome: Compartment syndrome occurs when the pressure increases within a fascial compartment which compromises blood flow and can result in tissue necrosis once the intra-compartmental pressure is greater than the arterial pressure. It commonly occurs following trauma and the anterior compartment in the leg is particularly at risk. Following ischaemia or reperfusion of an ischaemic leg, compartment syndrome may also occur. It presents with severe pain and tenderness in the affected compartment associated with pain on passive movement of the muscles, and later neurosensory loss. The treatment is fasciotomy to release the muscle; this should be performed following reperfusion if there is any question of compartment syndrome.
Cerebrovascular accident (CVA): although the limb may be pale, cool and paralysed it should not be painful and Doppler signals should be audible.
Deep vein thrombosis (DVT): the leg is usually warm, pink, swollen and tender. In phlegmasia cerulea dolens a DVT can cause venous gangrene. The foot usually appears blue, purple or black. Arterial Doppler signals should be audible. Phlegmasia cerulean dolens should also be referred to a vascular specialist.
Hypovolaemic: Shock can present with pulseless limbs, but an accurate history and examination (hypotension, all limbs affected) should clarify the diagnosis
Acute compressive neuropathy can present with a paralysed limb. Again Doppler signals should be normal.
Learning Bite
Arterial Doppler examination and an accurate history should differentiate acute limb ischaemia from other common differential diagnoses.
- ECG: To diagnose atrial fibrillation or other cardiac arrhythmias or an acute cardiac event, which may be a source of emboli.
- Bloods: Blood tests relevant to a suspected acutely ischaemic limb are listed in Table 3.
Table 3 Blood Tests for Acute Limb Ischaemia
| Investigation | Reason For Investigation |
| Full blood count | To detect haematological disorders predisposing to thrombosis |
| Urea and Electrolytes | Patients are often dehydratedPotassium may be raised if muscle necrosis has occurred |
| Glucose | To screen for diabetes |
| Creatinine Kinase | May be raised if muscle ischaemia has occurred |
| Clotting | It is rare to detect clotting abnormalities but it is usual to check before prescribing heparin |
| Group and Save | Patient may undergo surgery |
| Arterial Blood Gas analysis | To look for acidosis secondary to ischaemia. If the patient is acidotic the most important initial management is rehydration. |
Imaging
The urgency of imaging depends on the presentation.
Conventional imaging consists of a digital subtraction angiogram. This is an invasive procedure using intra-arterial contrast but has the potential for therapeutic intervention (thrombolysis, angioplasty).MR angiography and CT angiography are less invasive and should provide the same anatomical information. Arterial duplex is non-invasive but is operator dependent and iliac and calf vessels can be difficult to image. The choice of imaging is likely to depend on the local resources available.
Initial management in the Emergency Department
Analgesia: There is no specific analgesic for ischaemia, but anticipate the potential for high analgesic requirements particularly if there is an element of compartment syndrome, in which disproportionate pain to the clinical appearance is a feature. IV opiates are likely to be required as part of a multimodal approach. Patients with limb ischaemia are likely to be vasculopaths; NSAIDs may increase the risk of myocardial events and should not be used routinely. Neuropathic pain may sometimes be associated with critical limb ischaemia. However, no RCTs or observational studies comparing opioids, gabapentin, pregabalin, or tricyclic antidepressants with each other for the treatment of pain associated with critical limb ischaemia were identified in a 2012 search. As with any painful condition there is no rationale to withhold analgesia in order to facilitate assessment.
Oxygen: all patients should be administered supplemental oxygen. There are no specific trials of oxygen in acute limb ischaemia [13].
Heparin: 5000 units intravenous heparin (unfractionated) should be given immediately to all patients with acute limb ischaemia, even they are likely to be undergoing surgery or angiography [1]. This is to prevent propagation of thrombosis. In patients in whom definitive treatment is deferred an intravenous heparin infusion should be prescribed.
IV Fluids: Patients with acute limb ischaemia are often dehydrated. In addition they are likely to be undergoing surgery or being given iodinated contrast which will be a further renal insult. Reperfusion of ischaemic tissue releases toxic metabolites, potassium, creatinine kinase and myoglobin which can further damage the kidneys. Administration of potassium should be avoided.
Refer: Refer to a vascular specialist urgently. Any delay risks jeopardising the limb, particularly if there is sensorimotor impairment. Familiarity with local vascular centre policies is advisable; it is also important to be familiar with medication limitations for prehospital and transport services, who may not be able to transport patients on heparin infusions.
Learning Bite
All patients with acute limb ischaemia should receive analgesia, oxygen and heparin. All patients with acute limb ischaemia should be referred urgently to a vascular specialist.
Definitive management
This is based on Rutherfords Classification:
Category I These patients have a viable limb. They should be admitted, given analgesia and oxygen and heparinised (infusion). There is no good evidence to support the use of low molecular weight heparin in this situation and it is more difficult to adjust if interventions are required. Formal imaging (angiogram, MR angiogram, CT angiogram or arterial duplex depending on local resources) should then be arranged within normal working hours to plan definitive treatment.
Category IIa These patients have a threatened limb. They should be given oxygen, analgesia and heparin. Ideally they should have immediate imaging, in order to guide operative (or endovascular) intervention. In some cases where there is minimal sensory loss only, they may be managed conservatively overnight, and imaging obtained the following day.
Category IIb These patients have a threatened limb and cannot wait overnight. They should be given oxygen, analgesia and heparin. If circumstances allow it may be possible to obtain imaging prior to theatre, but this should not delay intervention. They need urgent revascularisation, either operatively or with thrombolysis (see below). Imaging may be acquired whilst the patient is in theatre.
In a patient with a clear history for embolus (and a source of embolus) and a normal contralateral limb, an embolectomy may be performed under local anaesthesia. The advantage of this is that local anaesthesia is better tolerated by elderly patients with cardiac comorbidity. The disadvantage is that if a simple embolectomy is unsuccessful and a more extensive procedure is required it may be necessary to convert to general anaesthesia. In all cases an anaesthetist should be present to manage the patient medically during the procedure. On-table imaging should be available and the whole leg prepared and draped.
Following revascularisation the limb may swell and the need for fasciotomy should always be considered.
Category III These patients have irreversible ischaemia and the limb is not salvageable. Revascularisation in this situation is likely to kill the patient, due to the massive release of potassium, creatine kinase, myoglobin, lactate and oxygen free radicals from the ischaemic tissue which can cause renal failure, myocardial toxicity and multi-organ failure. The options are either amputation or palliation. In an acute scenario with evidence of infection a so-called guillotine amputation may be performed in order to allow a quick operation to remove necrotic/infected tissue, with a definitive amputation at a later date once the patient is more stable and infection has been treated. It is vital to recognise those patients in which an ischaemic limb is part of the process of dying, and not subject them to unnecessary and futile interventions.
Thrombolysis
Intra-arterial thrombolysis is an alternative treatment to surgery for the acutely ischaemic limb. Intra-arterial streptokinase or rtPA (tissue plasminogen activator) convert plasminogen into plasmin, which lyses thrombin. Following thrombolysis an angiogram should be performed to identify any underlying stenosis.
There have been five randomised controlled trials comparing surgery with thrombolysis in the acutely ischaemic limb including 1283 patients, which have been analysed in a systematic review [9]. Major complications were more common after thrombolysis with a 1.3% risk of stroke (versus 0% after surgery) and an 8.8% risk of major haemorrhage (versus 3.3% after surgery). Whilst there was clinical heterogeneity between the studies the authors concluded that Universal initial treatment with either surgery or thrombolysis cannot be advocated on the available evidence . There have been no further randomized controlled trials since. In the UK thrombolysis use peaked in the late 1990s and most centres now use surgery as a first line management in most patients, mainly due to concerns regarding efficacy and complication rates for thrombolysis [15].
Interventional radiology approaches, including mechanical thrombectomy, have gained an evidence base in treatment of acute CVA. In patients presenting with ALI (<2 weeks of duration), endovascular and surgical approaches have similar rates of short-term and 12 month mortality, limb amputation and recurrent ischemia [10].
The contra-indications to thrombolysis are shown in Table 4:
| Bleeding or severe bleeding tendency |
| Pregnancy |
| CVA/TIA <2mths ago |
| Intracerebral tumour/AVM/aneurysm |
| Surgery <2 weeks go |
| Previous GI bleed |
| Trauma <10 days ago |
Table 4 Contra-indications to Thrombolysis
Learning Bite
There is no evidence to favour thrombolysis over surgery in the acutely ischaemic limb.
Acute upper limb ischaemia
Acute arm ischaemia is much less common than acute leg ischaemia (about 20% of all limb ischaemia [16]) and is usually due to cardiac emboli since atherosclerosis does not usually affect upper limb vessels. More rarely it can be due to emboli from a subclavian artery stenosis (clue: listen for subclavian bruit). Vasculitis and thoracic outlet syndrome can rarely cause an acutely ischaemic arm.
The commonest sites of occlusion are the axillary and brachial arteries.
The clinical assessment of the limb should follow the same principles as that for lower limb ischaemia, the aim being to identify limbs that need urgent intervention. As for patients with lower limb ischaemia, if there is no neuromuscular impairment patients can be admitted and heparinised overnight. Patients with signs of sensorimotor impairment should proceed to surgery. This will usually consist of a brachial embolectomy which can be performed under local anaesthesia, however occasionally more extensive procedures are required.
The initial management follows the same principles as for the acutely ischaemic leg: oxygen, analgesia and IV heparin. The patient should then be referred to a vascular specialist.
Figure 3 shows an MRA demonstrating emboli to the left hand, causing loss of the digital arteries to the lateral aspect of the index and middle fingers and the medial aspect of the ring and little fingers. This presented with cool pale 4th and 5th fingers.
Problems and Pitfalls
Popliteal aneurysms (See Figure 4) tend to accumulate thrombus. Because of their position behind the knee joint this can dislodge and embolise to the foot. Alternatively the aneurysm may occlude due to thrombosis. Any patient with a suspected popliteal aneurysm and ischaemic symptoms (even if these are only mild, since they may herald a more severe event) should be referred urgently to a vascular specialist; acute limb ischaemia due to a thrombosed popliteal aneurysm carries a 50% risk of amputation.
Figure 4: Large popliteal aneurysm
(ii) Palliative care Acute limb ischaemia may occur as a pre-terminal event. It is important to recognise this, in order that a patient who is dying may be managed appropriately and spared futile interventions (12).
(iii) Bilateral limb ischaemia this can be more difficult to diagnose, since both legs are abnormal. It may be caused by a saddle embolus at the aortic bifurcation (which carries a high (30%) mortality(13)) or can occur as a result of abdominal aortic dissection (this classically presents with back pain and hypotension).
Key Learning Points
- Acute limb ischaemia carries a high morbidity and mortality (level 4 evidence)
- Few patients present with simple embolus without underlying peripheral arterial disease (level 4 evidence)
- The classical signs of acute limb ischaemia may be attenuated in a patient with underlying peripheral arterial disease due to the development of collateral vessels (level 5 evidence)
- All patients with suspected acute limb ischaemia should have arterial Doppler examination performed (level 5 evidence)
- All patients with acute limb ischaemia should receive analgesia, heparin and oxygen (level 5 evidence)
- Assessment of sensorimotor deficit helps determine the urgency of intervention (level 4 evidence)
- Patients with no or mild sensory loss should proceed to formal imaging (usually angiography) prior to intervention (level 5 evidence)
- Patients with motor deficit may proceed to theatre for intervention with on-table imaging (level 5 evidence)
- Patients with fixed skin mottling and complete paralysis have signs of an unsalvageable limb: in these patients revascularisation is dangerous and the choice is between amputation and palliation (level 4 evidence)
- There is no evidence to support the use of thrombolysis over surgery in the management of the acutely ischaemic limb (level 1a evidence)(9)
- Beware the patient with a popliteal aneurysm and a history of acute limb ischaemia: a thrombosed popliteal aneurysm carries a 50% risk of amputation (level 4 evidence)
- National Institute for Health and Care Excellence. Peripheral arterial disease: diagnosis and management. NICE guideline [CG147]. Published: 08 August 2012, Updated: 11 December 2020.
- Jivegård L, Wingren U. Management of acute limb ischaemia over two decades: the Swedish experience. Eur J Vasc Endovasc Surg. 1999 Aug;18(2):93-5.
- Howard DP, Banerjee A, Fairhead JF, Hands L, Silver LE, Rothwell PM; Oxford Vascular Study. Population-Based Study of Incidence, Risk Factors, Outcome, and Prognosis of Ischemic Peripheral Arterial Events: Implications for Prevention. Circulation. 2015 Nov 10;132(19):1805-15.
- Fowkes F, Housley E, Cawood E, Macintyre C, Ruckley C, Prescott R. Edinburgh Artery Study: prevalence of asymptomatic and symptomatic peripheral arterial disease in the general population. Int J Epidemiol 1991; 20(2):384-392.
- Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, C et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001 Sep 19;286(11):1317-24.
- Aune S, Trippestad A. Operative mortality and long-term survival of patients operated on for acute lower limb ischaemia. Eur J Vasc Endovasc Surg. 1998 Feb;15(2):143-6.
- Eliason J, Wainess R, Proctor M, Dimick J, Cowan J, et al. A National and Single Insitutional Experience in the Contemporary Treatment of Acute Lower Extremity Ischaemia. Ann Surg 2003; 238(3):382-390.
- Campbell WB, Ridler BM, Szymanska TH. Current management of acute leg ischaemia: results of an audit by the Vascular Surgical Society of Great Britain and Ireland. Br J Surg. 1998 Nov;85(11):1498-503.
- Berridge DC, Kessel D, Robertson I. Surgery versus thrombolysis for acute limb ischaemia: initial management. Cochrane Database Syst Rev. 2002;(3):CD002784. doi: 10.1002/14651858.CD002784. Update in: Cochrane Database Syst Rev. 2013;6:CD002784.
- Enezate TH, Omran J, Mahmud E, Patel M, et al. Endovascular versus surgical treatment for acute limb ischemia: a systematic review and meta-analysis of clinical trials. Cardiovasc Diagn Ther. 2017 Jun;7(3):264-271.
- Kuukasjarvi P, Salenius J, Finnvasc study group. Perioperative Ouctome of Acute Lower Limb Ischaemia on the Basis of the National Vascular Registry. Eur J Vasc Endovasc Surg 1994; 8:578-583.
- Campbell WB, Verfaillie P, Ridler BM, Thompson JF. Non-operative treatment of advanced limb ischaemia: the decision for palliative care. Eur J Vasc Endovasc Surg. 2000 Mar;19(3):246-9.
- Mercer K, Berridge D, Golden M. Saddle embolus-the need for intensive investigation and critical evaluationq. Vascular Surgery 2001; 35(1):63-65.
- Berridge DC, Hopkinson BR, Makin GS. Acute lower limb arterial ischaemia: a role for continuous oxygen inhalation. Br J Surg. 1989 Oct;76(10):1021-3.
- Richards T, Pittathankal AA, Magee TR, Galland RB. The current role of intra-arterial thrombolysis. Eur J Vasc Endovasc Surg. 2003 Aug;26(2):166-9.
- Eyers P, Earnshaw JJ. Acute non-traumatic arm ischaemia. Br J Surg. 1998 Oct;85(10):1340-6.







33 Comments
comprehensive and useful informative module. Thanks.
Extremely useful and easy to understand. Highly recommend for new learner.
Great module
Very useful points
Frequently seen in ED, explained very well
Great workshop
good overview
Excellent
This module provides clear approach to manage ED presentation of Acute limb ischemia
Good module – very comprehensive.
excellent article
very useful
Great module
Useful/great module
useful revision
Good Revision
good read
Precise and easy to read.
Good account
Comprehensive interesting topic.
really nice
Good overview
Very useful informations about limb ischaemia.
Excellent; easy to follow and comprehend.
Useful
Concise presentation and useful learning bite.
Very comprehensive and systematic approach for assessment and management of patients with acute limb ischemia. Excellent learning module.
Very useful and important Module
very educative. useful module for ED clinicians
very useful and good learning points
I learned so much. I am more ready in looking after patients with potentially acute limb ischaemia. Thank you so much.
Very helpful module which will assist in the management of patients with acute limb ischaemia
Good overview