Author: Frances Balmer / Editor: Jason Kendall / Reviewer: Rebecca Ford, Frances Balmer / Codes: AC1, PhC3, PhC4, PhP1, RP2, SLO3 / Published: 16/05/2022
Context
Hypoxia is common in patients presenting to the ED and can be life-threatening. The differentials are wide and include cardiac, respiratory and haematological causes.
Methaemoglobinaemia accounts for only a small proportion of these cases and so the diagnosis is often delayed or missed. A thorough history, examination and analysis of investigations leads to the diagnosis. Early treatment can reverse methaemoglobinaemia within the hour, reducing subsequent morbidity and mortality. As such, it is essential for the ED physician to be familiar with the presentation and management of this uncommon but potentially reversible condition.
Learning Bite
Methaemoglobinaemia is an uncommon but fully reversible cause of hypoxia – early diagnosis and treatment is essential.
Definition
Methaemoglobinaemia is a disorder characterised by a higher than normal level of methaemoglobin (MetHb) in the blood [1, 2].
In health, MetHb is < 1% (of total haemoglobin)
Physiology of haemoglobin
Red blood cells contain haemoglobin (Hb). Haemoglobin binds oxygen when partial pressures of oxygen are high (for example at the lungs) and releases it when partial pressures of oxygen are low (for example at body tissues). It is responsible for the majority of oxygen transport in the body.
The structure of haemoglobin is adapted to it’s function. Haemoglobin is a tetramer of four proteins (globins) each of which carry a haem group. Haem consists of an iron molecule held in a porphyrin ring structure. It is the iron molecule which binds oxygen, thus each haemoglobin molecule has the capacity to carry four oxygen molecules. In addition the binding of one oxygen molecule causes a structural or conformational change in the haemoglobin molecule which increases the affinity of the remaining haem units for oxygen. This cooperativity gives rise to the classical sigmoid-shaped oxygen-dissociation curve.
Ferrous and Ferric iron
The majority of iron in haem is in the form of Fe2+ (ferrous ion); this functional form can bind oxygen. Fe2+ can be oxidised to Fe3+ (ferric ion); Fe3+ is non-functional and cannot bind oxygen. Haemoglobin containing Fe3+ is called Methaemoglobin (MetHb).
Furthermore the presence of Fe3+ alters the structure of the whole haemoglobin molecule to increase the affinity of the remaining Fe2+ ions for oxygen (i.e. a left shift of the oxygen-dissociation curve).
Therefore the presence of Fe3+ has a dual impact on oxygen carriage, haemoglobin can carry less oxygen AND oxygen release at the tissues is impaired causing a more profound tissue hypoxia than indicated by the SaO2.
When protective mechanisms fail, methaemoglobinaemia results
Red blood cells are continuously exposed to oxidative stresses which convert haemoglobin to MetHb. In health, levels of MetHb are maintained at < 1% by a host of protective reduction pathways that restore Fe3+ to Fe 2+.
Foremost of these is the cytochrome-b5-reductase (also called NADH-methaemoglobin-reductase) pathway. This pathway is responsible for 99% of MetHb reduction under normal conditions.
Other minor pathways involve other reducing agents including NADPH-methaemoglobin-reductase (which requires functioning G6PD), ascorbic acid and glutathione. These minor pathways offer options for the treatment of methaemoglobinaemia, as will be discussed later.
When the physiological balance of iron oxidation and reduction is upset, either by an increase in oxidative stresses (causing increased MetHb production) or by ineffective reduction (causing reduced clearance of MetHb), methaemoglobinaemia results and oxygen delivery falls.
Learning Bite
Hb can be oxidised to MetHb. MetHb does not carry oxygen efficiently. In health, protective enzymes reverse Hb oxidation to keep MetHb levels at < 1%. The major enzyme involved is cytochrome-b5-reductase. When these protective mechanisms fail, MetHb levels rise and hypoxia results.
Causes of methaemoglobinaemia
Methaemoglobinaemia can be innate (e.g. a genetic deficiency of enzymes involved in reduction pathways or altered haemoglobin structure) or acquired (e.g. exposure to toxins that increase oxidation reactions). Some cases are idiopathic [1].
Table 1: Causes of methaemoglobinaemia
| Cause | Notes | |
| Acquired | Toxins | |
| Diet | Historically well water contaminated by fertiliser was a common cause | |
| Endogenous states of increased oxidative stress | E.g. Sepsis | |
| Metabolic acidosis? | There is an association between methaemoglobinaemia and metabolic acidosis due to dehydration in young children with diarrhoea and vomiting. This is thought to be due to reduced action of cytochrome-b5-reductase in acidic conditions [3]. | |
| Innate | Cytochrome-b5-reductase deficiency | Loss of protective mechanism |
| Pyruvate kinase deficiency | Reduced NADH production. NADH is a cofactor for cytochrome-b5-reductase | |
| Haemoglobinopathy (HbM) | Genetic mutation replaces a histidine residue with a tyrosine residue which alters the structure of the globin proteins and limits the ability of reducing enzymes to access Fe3+ | |
| Idiopathic | ||
In adults or older children with no previous history of methaemoglobinaemia, the most common cause is exposure to a toxin. The toxins may act directly as oxidising agents or indirectly by producing oxygen and peroxide free radicals which in turn oxidise Fe2+. The effect is variable depending on dose, timing of exposure and variability in metabolism between individuals.
Many, many, many, many toxins are implicated
The list of causative toxins reported in the literature is exhaustive but of note are local anaesthetics, nitrites (including alkyl or amyl nitrites or poppers), Metoclopramide and antibiotics including Dapsone [1, 2].
Table 2: Some of the commoner toxins associated with methaemoglobinaemia
| Aniline (dyes, inks) | Benzocaine | Chlorates | Chloroquine |
| Dapsone | Hydroxylamine | Lidocaine | Metoclopramide |
| Methylene Blue | Nitrates | Nitric oxide | Nitrites |
| Nitroglycerin (GTN) | Paraquat | Phenols | Phenytoin |
| Prilocaine | Smoke inhalation | Sulfonamide | + many more |
Learning Bite
The most common cause of methaemoglobinaemia is toxin exposure taking a thorough history is essential. In young children, congenital causes should also be considered
The patient presents with
Methaemoglobinaemia can be asymptomatic or cause a range of hypoxic symptoms.
Table 3: Symptoms related to percentage MetHb
| MetHb as % of total Hb* | Typical Symptoms+ |
| <10 | Symptoms unlikely |
| 10-20 | Cyanosis (blue/grey) |
| 20-30 | Anxiety, headache, light headedness, tachycardia |
| 30-50 | Fatigue, confusion, dizziness, tachycardia, tachypnoea |
| 50-70 | Coma, seizures, arrhythmias, respiratory depression |
| > 70 | Death |
* for a Hb of 15 g/dL
+ symptom severity does not always reliably correlate to MetHb level but is affected by individual factors including pre-existing co-morbidities
Cyanosis is usually the first sign and patients can initially appear very well for the level of cyanosis. This is because cyanosis secondary to methaemoglobinaemia appears at 1.5 g/dL of MetHb, whereas cyanosis secondary to hypoxaemia appears at 5 g/dL of deoxygenated haemoglobin, which represents a much greater reduction in oxygen carrying capacity.
Patients often have a low SpO2 on pulse oximetry but again appear well for the degree of hypoxia. A notable feature of methaemoglobinaemia is a low SpO2 on pulse oximetry which does not improve with supplemental oxygen. This can be the first pointer to the diagnosis of methaemoglobinaemia [4].
Learning Bite
In any patient who presents with cyanosis or low SpO2 out of keeping with their clinical state, and which does not improve with supplemental oxygen, think methaemoglobinaemia.
Initial assessment history and examination
Initial assessment should follow an ABCDE approach as for every hypoxic patient, supporting ventilation and circulation and identifying and treating other causes of cyanosis or other effects of toxin exposure. Anaesthetics or ITU input may be required.
If methaemoglobinaemia is suspected there are key points of the history and examination that should be elicited to aid diagnosis, identify cause and allow assessment of severity.
The most important part of the history is to elicit any history of exposure to account for the methaemoglobinaemia. It is also necessary to identify co-morbidities and any history of cyanosis or haematological abnormality.
Examination should look for evidence of compromise (i.e. signs of hypoxia) and any clues as to the cause including presence of sepsis
Learning Bite
Be thorough in eliciting a history of exposure to toxins it is the most common cause of methaemoglobinaemia and allows avoidance of that agent in future
Initial assessment bedside tests
Bedside tests also provide useful information.
1) Chocolate Blood: In methaemoglobinaemia, arterial blood is chocolate coloured and it does not oxidise or turn redder on exposure to air
Normal blood sample on left compared to chocolate blood on right
2) Brown / black urine: Urine can also be discoloured
3) A low SpO2 on pulse oximetry which does not improve with supplemental oxygen. SpO2 as measured by pulse oximetry usually does not fall lower than 85%, so significantly lower readings suggest an alternative or additional cause of hypoxia.
MetHb: The gold standard investigation is a MetHb level which may be available as standard on ABG co-oximetry or may have to be specifically requested.
ABG: Will show a normal PaO2 (or raised if receiving supplemental oxygen) and reduced SaO2*. This allows calculation of the Saturation Gap.
* some older ABG machines calculate SaO2 from SpO2, pH and bicarbonate. These machines give a falsely high reading for SaO2 as they assume Hb is normal. Most modern ABG machines are combined with a co-oximeter which measures light absorption at four (or more) wavelengths and allows direct measurements of COHb (600nm) and MetHb (631nm). Thus they give an accurate SaO2 and MetHb.
FBC: Check Hb levels. MetHb will be less well tolerated in anaemia.
ECG: Rule out differentials or contributory causes. MetHb can cause arrhythmias secondary to hypoxia. Toxin exposure can also cause conduction disturbances.
CXR: Rule out differentials or contributory causes to hypoxia
bHCG: Pregnancy has implications for treatment thresholds and the need for full discussion of risks and benefits to gain informed consent
Learning Bite
Get an ABG early for accurate SaO2 and MetHb level.
Low risk patients can be treated with supportive care and observation whilst high risk patients require active treatment to reverse the methaemoglobinaemia [5].
Traditionally active treatment is only indicated if:
- MetHb > 30% regardless of whether symptomatic or not
or
- MetHb < 30% and symptomatic
Some authors challenge this threshold as MetHb is not the only indicator of disease severity [6]. Significant variation exists between individuals and additional factors should be considered when stratifying risk:
1) Evidence of tissue hypoxia due to MetHb (e.g acidosis, end-organ dysfunction, altered mental state, myocardial ischaemia or arrhythmia).
2) Co-morbidities. Pre-existing cardiovascular, respiratory and haematological conditions or acute conditions (e.g. sepsis) which hinder oxygen delivery will reduce the threshold for symptoms and active treatment.
3) Hb level. Anaemic patients will be symptomatic at a lower concentration of MetHb.
Two patients with a MetHb of 1.5 g/dL will both be cyanotic. But if one has a Hb of 15 g/dL (i.e. 10% MetHb) they will be otherwise well, whereas if the other has a Hb of 7.5 g/dL (i.e. 20% MetHb) they will be symptomatic.
4) Chronic or acute methaemoglobinaemia. A low level methaemoglobinaemia can be normal for a patient with cytochrome-b5-reductase deficiency and not require emergency treatment. Furthermore patients with a chronic methaemoglobinaemia will have developed compensatory mechanisms and have a degree of tolerance lacking in naive patients [1].
Learning Bite
- Active treatment is indicated for patients with MetHb > 30% and those who have evidence of tissue hypoxia due to methaemoglobinaemia.
- Risk assessment should not end at MetHb level but also consider pre-existing and active conditions.
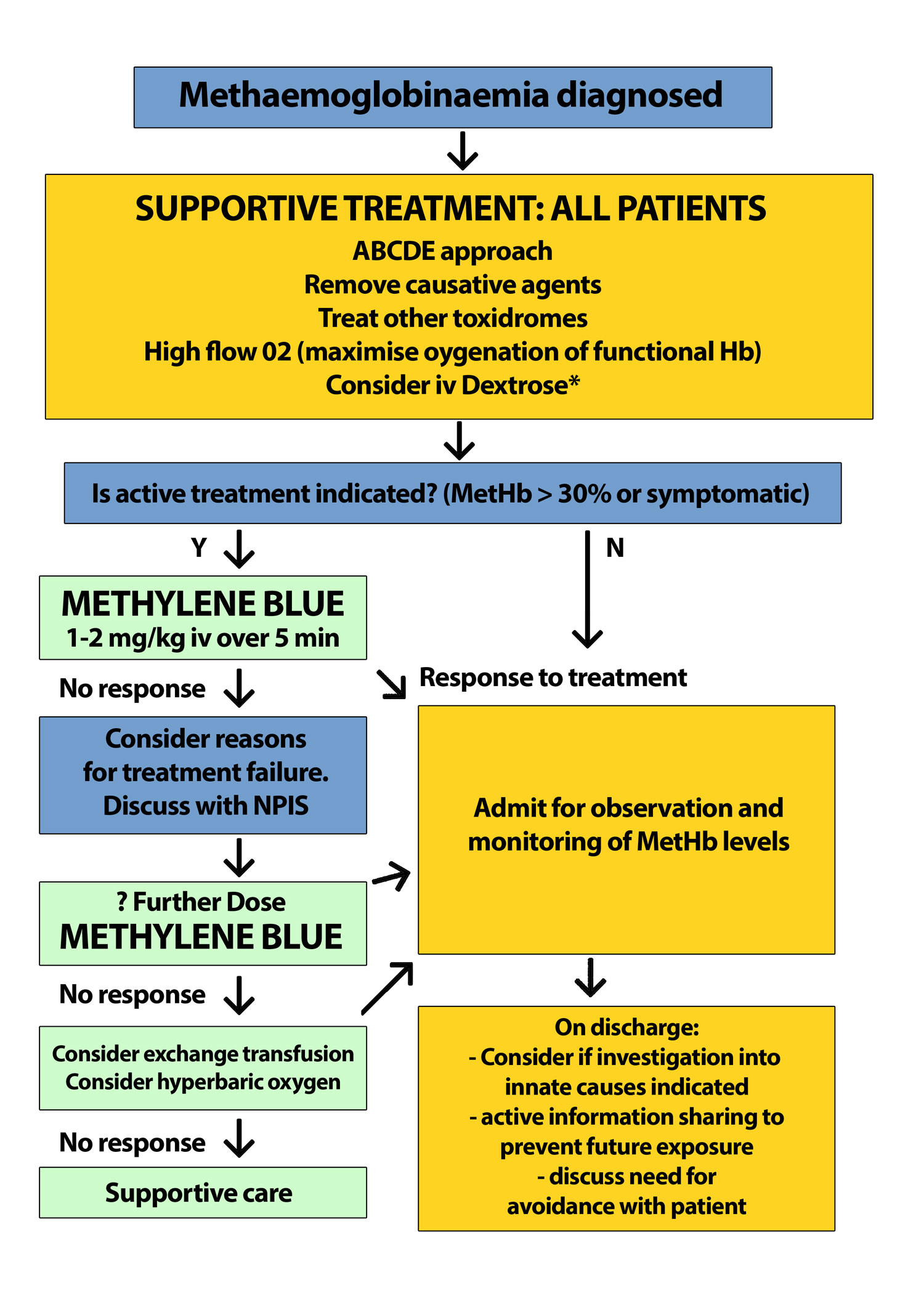
Treatment pathway
If there are any concerns about treatment, contact the National Poisons Information Service.
A guide to treatment is as follows:
*Dextrose there is no trial evidence per say for the use of intravenous Dextrose but its use is well described and biologically sound; both NADH and NADPH are produced by the metabolism of glucose [1].
Other therapeutic options including high dose ascorbic acid, cytochrome P450 inhibitors, and N-acetylcysteine have been proposed but there is as yet insufficient robust evidence to recommend their use [7, 8].
Methylene Blue [5,9]
Methylene Blue is an aromatic dye found in the antidote cupboard of the ED.
Action: Acts as a co-factor in a MetHb reducing reaction. NADPH-methaemoglobin-reductase reduces Methylene Blue to Leukomethylene blue, which in turn reduces Fe3+ to Fe2+. Thus this pathway, which only plays a minor role in health, can be exploited to become the main therapeutic target in methaemoglobinaemia.
Administration: 1-2 mg/kg iv over 3-5 minutes
Response: Effects should be seen quickly with a dramatic reduction in MetHb in < 30 minutes.
If required further doses can be given after 30 minutes (discuss doses over 4mg/kg with local poisons service)
Monitoring: Methylene Blue interferes with pulse oximetry so SpO2 will appear to fall with treatment (and can go as low as 65%). Monitoring is by co-oximeter measurement of MetHb and SaO2.
Side effects: Nausea and vomiting, blue urine, diaphoresis, restlessness, anaphylaxis, pulmonary hypertension, serotonin syndrome and haemolytic anaemia are reported.
Caveats and cautions to consider :
Dosage: Methylene Blue is actually an oxidative agent. In high doses the oxidative effects of Methylene Blue overcome the reductive effects of Leukomethylene blue and increase MetHb.
NADPH reductase and G6PD deficiency: Methylene Blue works via the NADPH pathway so will have no effect in patients with NADPH-methaemoglobin-reductase deficiency and limited effect in G6PD deficiency (G6PD catalyses the formation of NADPH). There are also documented reports of Methylene Blue causing haemolytic anaemia in G6PD deficiency [10].
Chlorate poisoning: Chlorate can inactivate G6PD.
M Haemoglobin: Methylene Blue has limited effect in patients with M Haemoglobin.
Sulfhaemoglobinaemia: MetHb can be been further modified by binding irreversibly to sulphur to form Sulfhaemoglobin (SulfHb). This happens with exposure to certain toxins, notable examples being Sulfasalazine and Sumatriptan. Clinically the presentation is similar. Patients may have a green tinge to their blood on venepuncture. There is no specific treatment. Sulfhaemoglobinaemia does not respond to Methylene Blue
Pregnancy: Very limited data exists but the general consensus is that the risks of treatment with Methylene Blue are less than withholding treatment if it is clinically indicated. Women should be counselled about the risks and benefits and have fetal monitoring throughout.
Paediatric focus – Infants presenting with cyanosis
The differential of cyanosis in children is huge and includes cardiac, circulatory, respiratory and haematological disease, which need to be identified and treated as appropriate.
Infants are a special case in methaemoglobinaemia because innate causes usually present early in life. That does not mean that environmental exposure can be forgotten. A careful history is required. Young children are at higher risk of developing acquired methaemoglobinaemia after an exposure as:
- they have lower levels of cytochrome-b5-reductase
- fetal Hb is more easily oxidised than adult Hb
- the infant gut contains higher levels of organisms that convert dietary nitrates to nitrites
- infants are more susceptible to dehydration and acidosis in diarrhoea and vomiting. It is proposed that acidosis inhibits the reductase pathways.
Suggested approach to the evaluation of cyanosis in neonates and infants
Remember innate Haemoglobin M, G6PD or NADPH-methaemoglobin-reductase deficiency will not respond to Methylene Blue in these infants but acquired causes (e.g. toxin exposure, dietary exposure) and cytochrome-b5-reductase deficiency will.
- The diagnosis of methaemoglobinaemia is often delayed. Top tips that should make you think MetHb:
- cyanosis or hypoxia on pulse oximetry which does not respond to supplemental O2
- a patient who is tolerating their hypoxia better than they should be
- chocolate brown coloured arterial blood
- a history of exposure to substances with oxidative capacity
- saturation gap the SpO2 and SaO2 are different by > 5%
- The agent which has caused methaemoglobinaemia may also have other toxic effects which require specific treatment. Consult Toxbase or the NPIS for further advice.
- Consider the whole patient (presentation, co-morbidities, extent of exposure) not just their MetHb level when deciding on active treatment.
- Pulse oximetry is not accurate in methaemoglobinaemia and paradoxically worsens in treatment with Methylene Blue do not be led by the SpO2.
- Methylene Blue does not work in NADPH-methaemoglobin-reductase deficiency, G6PD deficiency, the presence of haemoglobin M or Sulfhaemoglobin.
- Infants presenting with cyanosis may be having their first presentation of innate methaemoglobinaemia
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med. 1999 Nov;34(5):646-56.
- Cortazzo JA, Lichtman AD. Methemoglobinemia: a review and recommendations for management. J Cardiothorac Vasc Anesth. 2014 Aug;28(4):1043-7.
- Hanukoglu A, Danon PN. Endogenous methemoglobinemia associated with diarrheal disease in infancy. J Pediatr Gastroenterol Nutr. 1996 Jul;23(1):1-7.
- Ralston AC, Webb RK, Runciman WB. Potential errors in pulse oximetry. III: Effects of interferences, dyes, dyshaemoglobins and other pigments. Anaesthesia. 1991 Apr;46(4):291-5.
- National Poisons Information Service (NPIS). Methylthioninium chloride antidote. Available here. [L accessed on 04/11/2019].
- El-Husseini A, Azarov N. Is threshold for treatment of methemoglobinemia the same for all? A case report and literature review. Am J Emerg Med. 2010 Jul;28(6):748.e5-748.e10.
- Rino PB, Scolnik D, et al., Ascorbic acid for the treatment of methemoglobinemia: the experience of a large tertiary care pediatric hospital. Am J Ther. 2014 Jul-Aug;21(4):240-3.
- Wright RO, Magnani B, Shannon MW, Woolf AD. N-acetylcysteine reduces methemoglobin in vitro. Ann Emerg Med. 1996 Nov;28(5):499-503.
- Ferris D. BestBet : Methylene blue as a treatment for methaemoglobinaemia. 2010 Available here. [Last accessed Aril 2022]
- Youngster I, Arcavi L, Schechmaster R, Akayzen Y, Popliski H, Shimonov J, Beig S, Berkovitch M. Medications and glucose-6-phosphate dehydrogenase deficiency: an evidence-based review. Drug Saf. 2010 Sep 1;33(9):713-26.
- National Poisons Information Service (NPIS). Toxbase. Methaemoglobinaemia. [Last accessed 04/11/19].














11 Comments
Very interesting topic and well presented.
Practical approach to a situation which needs more consideration ED
Very interesting i have been lucky/unlucky to come across this twice in the last 10 years working in ED.
interesting topic
Great e-learning for a rare and easily missed cause of cyanosis/hypoxia. Thanks.
Interesting topic. Easy to miss… Thanks
A GREAT GUIDING PATHWAY WITH EMPHASIS ON WHAT TESTS TO CONDUCT AND WHAT MEDICATIONS TO BEGIN
very interesting. Using the concept of the saturation gap , gives something to consider
Thanks, great revision for practice and exams.
Good revision
Nice fruitful topic