Author: Cliff Mann / Editor: Jason Kendall / Reviewer: Martin Dore / Codes: CC10, CC3, CP1, CP3, SLO1 / Published: 15/01/2019
Atrial Fibrillation affects a substantial number of patients. It thought to effect up to 3% of the population and the incidence increases with age and co-morbidities such as hypertension, heart failure, coronary artery disease, valvular heart disease, obesity, diabetes
mellitus, and chronic kidney disease. 1
The greatest morbidity and mortality associated with AF arises from the thromboembolic sequelae however significance of the dysrhythmia is highlighted by the associated odds ratios for death. In men with AF in comparison to non AF controls the Odds Ratio for death is 1.5 and in women 1.9. Most of the excess mortality occurs early after the diagnosis of AF.
The management of atrial fibrillation is seldom entirely straightforward; the rate versus rhythm debate and the efficacy and safety of anticoagulation are just two examples of key decisions the clinician and patient must face. This situation is often compounded in patients presenting with AF to the Emergency Department as there may be uncertainty regarding onset time and relevance to the presenting complaint.
Definition
Atrial Fibrillation (AF) is an atrial tachydysrhythmia characterised by predominantly uncoordinated atrial activation with consequent deterioration of atrial mechanical function.
The p waves which represent depolarisation of the atria, are absent during atrial fibrillation and the heart rhythm is irregularly irregular.
The currently recognised classification of AF relates to the duration and persistence of the AF:
- Paroxysmal AF: AF which terminates spontaneously within seven days of onset and most often within 48 hours of onset.
- Persistent AF: AF present continuously for seven days or more or terminated by cardioversion.
- Permanent AF: AF which is accepted without attempted cardioversion or which cannot be terminated by cardioversion.2
Many of the patients who present to the Emergency Department in AF have had the condition diagnosed previously and been commenced on appropriate treatment, ie rate or rhythm control. However some will not previously been known to be in AF and will therefore require a full assessment with a view to commencing therapy.
(i) Assessment of time of rhythm onset
The most important ED determination in AF is the probable duration of the dysrhythmia. It is important as it allows the clinician to determine whether a rate or rhythm control strategy is in the best interest.
A safe time frame for cardioversion is 48 hrs from onset
Some patients present with what has often been called Fast AF. This is a misnomer since all patients in AF have chaotic atrial electrical activity with no discernible pattern, so the description fast which implies a contradistinction to slow is incorrect. The correct description is AF with a fast /slow / controlled ventricular response.
(ii) Assessment of precipitating events
There are many illnesses which may precipitate new atrial fibrillation or worsen the cardiovascular consequences of pre-existing AF.
These secondary causes can be remembered by the mnemonic:
PIRATES
PE,
Ischemia,
Respiratory disease,
Atrial enlargement or myxoma,
Thyroid disease (check TSH and free T4 in first-time presenters),
Ethanol (“Holiday heart” after binging) 3
Sepsis or Sleep apnea
Learning Bite
Where there are identifiable precipitants of atrial fibrillation they should be treated. There has been shown to be 6 fold increase in adverse event rate when treating AF secondary to an acute underlying medical illness in the emergency department. 4 The AF usually improves or resolves if treatment is directed at the precipitant.
(iii) Assessment of Haemodynamic Consequence
Most patients with AF are cardiovascularly stable. Two uncommon groups of patients may become unstable as a consequence of the onset of AF.
- Those patients in whom adequate LV function is dependent upon the 15% of ventricular filling provided by atrial contraction i.e. patients with poor LV function
- Patients in whom the ventricular response to AF results in very high heart rates (>150 bpm). In such cases there is inadequate time for LV filling with a consequent reduction in LV output and this is exacerbated by a reduction in time in diastole with a consequent reduction in coronary blood flow. This may exacerbate ischaemia and further compromise LV function.
In both groups restoration of sinus rhythm assumes increased importance and prompt cardioversion may be indicated. However for patients in the second group rate reduction may be sufficient.
(iv) Assessment of Stroke Risk
The greatest morbidity and mortality arising from AF is caused by thromboembolic sequelae. It follows that assessment and reduction of this risk is the single most important aspect of the treatment of AF.
All patients presenting with atrial fibrillation should be subject to a formal stroke risk assessment using the CHA2DS2-VASc score (Fig 1)
NICE recommends treatment must be decided on an individual basis:
- Consider anticoagulation for men with a CHA2DS2-VASc score of 1
- Offer anticoagulation to people with a CHA2DS2-VASc score of 2 or above
Bleeding risk should be assessed using the HAS-Bled Score (Fig 2), using a score of greater than 2 as a relative contraindication to anticoagulation.
Figure 2
When deciding treatment the expected stroke reduction, bleeding risk, and patient preference should be considered.
Basic science and pathophysiology
The primary pathological change seen in atrial fibrillation is progressive atrial fibrosis. This fibrosis is usually associated with dilation of the atria and can occur as a result of a number of mechanisms including valvular and ischaemic heart disease. As a result of the dilation and fibrosis the atria undergo physical and electrical remodeling. The longer AF is present the greater and more irreversible these changes become.
There are two main explanations thought to contribute to the prothombotic state experienced in patients with AF.
1. Structural and functional changes in the atrial myocardium along with stasis of blood especially in the Left atrial appendage allow an environment in which throbus can form.
2. Damage to the myocardium , even seen with short episodes of AF cause expression of prothrombotic factors on the atrial endothelial surface. This along with activation of platelets and inflammatory cells leads to a clot formation.1
This can explain why even those presenting with paroxysmal AF or a brief episode should be considered for stroke risk assessment.
Investigation
All patients with suspected AF must undergo a physical examination, 12 lead ECG and a blood sample sent for routine haematological and biochemical analysis (electrolytes and thyroid function are important considerations)
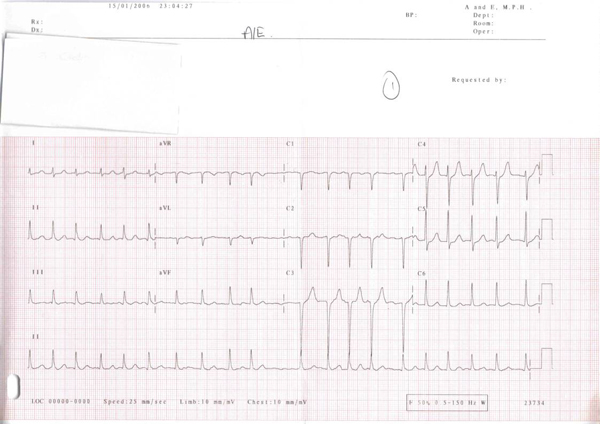
The hall-mark feature of the ECG of a patient in AF is the presence of an irregularly irregular rhythm as shown on the ECG in Figure 3.
Figure 3: 12 lead ECG of AF note irregularly irregular rhythm and lack of p waves
Differential Diagnosis
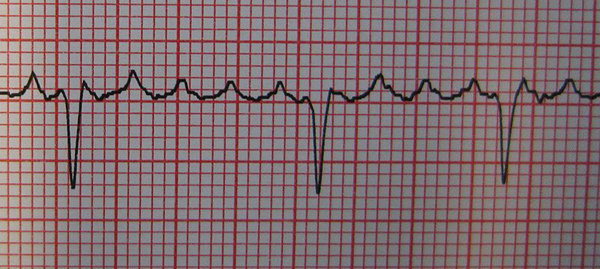
The differential diagnoses are atrial flutter with variable block (Figure 4) and multifocal atrial tachycardia (Figure 5).
Figure 4: Atrial flutter with variable block note the characteristic saw-tooth p waves often seen best in lead V1
Figure 5: Multifocal atrial tachycardia most commonly seen in association with severe pulmonary disease.
The key features are an atrial rate in excess of 100bpm and at least 3 morphologically different non-sinus p waves in the same ECG lead.
The main goals of treatment of atrial fibrillation are to minimise circulatory instability or insufficiency and to prevent stroke.
Circulatory instability or insufficiency are normally managed by either a rate or rhythm control strategy. In an emergency, when circulatory collapse is imminent immediate cardioversion may be indicated.
In the ED the approach to the patient in AF is determined primarily by the duration of the rhythm. In patients in whom the time of AF onset can be determined to be within 48hrs of presentation restoration of sinus rhythm is both safe and desirable.
Consider either pharmacological or electrical cardioversion depending on clinical circumstances and resources.
Electrical
Synchronised direct current (DC) cardioversion.
Unless the patient is unconscious safe sedation/GA should be undertaken as per local protocol and a starting voltage of 120-150J should be delivered, the energy can be increased in increments if this fails.56
Pharmacological
Flecainide has the highest success rate with respect to restoration of sinus rhythm and is significantly quicker in onset than amiodarone, however it is contraindicated in patients with ischaemic or structural heart disease.6
When conducting pharmacological cardioversion in the ED:
A choice of flecainide or amiodarone should be used in people with no evidence of structural or ischaemic heart disease.
Amiodarone should be used in people with evidence of structural heart disease.
After 48hrs there is ample evidence that restoration of sinus rhythm risks dislodging thrombi from the left atrial appendage with the consequent risk of thromboembolic events including stroke.
In patients presenting after 48 hours of symptom onset or where there is doubt as to when the AF began the ED approach is limited to determining the stroke risk and controlling the ventricular rate.
The management for these patients in the emergency department consists of rate control and anticoagulation dependant on stroke risk assessment.
Rate control, slowing the heart, increases the proportion of time and the amount of time spent in diastole thereby increasing the time available for coronary blood flow. Similarly, by increasing the time spent in diastolic filling of the ventricles, the cardiac output is increased.
A Standard B-blocker or rate limiting calcium channel blocker should be used with a target heart rate of less than 110bpm. 7
Rhythm control should be considered in all patients with persistent AF in whom there are no contra-indications. In most circumstances relevant to the ED this will require commencement of anticoagulation and referral to cardiology (often through the patients GP) for an elective interval cardioversion after 3 weeks of anticoagulation. During this interval it may be necessary to control the ventricular rate.
Although counterintuitive, for many patients restoring sinus rhythm has not been shown to provide morbidity or mortality benefits.
NICE recommends rate control as the first-line strategy to people with atrial fibrillation, except in people:
- Whose atrial fibrillation has a reversible cause
- Who have heart failure thought to be primarily caused by atrial fibrillation
- With new-onset atrial fibrillation (<48hrs)
- For whom a rhythm control strategy would be more suitable based on clinical judgement.
Anticoagulation
The choice of anticoagulant, if deemed necessary, will be locally decided but Warfarin is being replaced nationally by the use of NOACs for the majority of patients.
Learning Bite
No study has found rate control to be inferior to rhythm control
Haemodynamically unstable AF
Rarely but importantly patients may present with haemodynamic compromise secondary to AF. Such instability due to AF rarely occurs unless the ventricular rate remains above 150 bpm for prolonged periods. It may be associated with hypotension or ischaemic chest pain. In such cases unless the AF is known to be long standing DC cardioversion is the safest and most effective treatment option. Where the AF is longstanding the choice of agent will be governed by the presenting features. Where the main feature is ischaemic (presumably rate related) chest pain a B-blocker is the most rational choice, where the main feature is hypotension a B blocker is contraindicated and therefore amiodarone would be a more rational choice.
In patients with an accessory pathway (e.g. WPW syndrome), the onset of AF may bring about profound hypotension. This occurs because the accessory pathway can conduct the fibrillation waves
much more rapidly than the AV node. Nodal conduction is limited to approximately 180bpm whereas in patients with accessory pathways the rate may rise to almost 300 bpm. At such rates there is virtually no time for ventricular filling with a consequent precipitous fall in cardiac output. It is for this reason that AV nodal blocking agents should never be given to patients in AF where an accessory pathway is suspected.
An algorithm summarising the treatment of AF in the ED is shown below.
Mis-diagnosis of Atrial Fibrillation:
- Where doubt exists a long rhythm strip is required.
- Remember the faster the rate the more regular AF will look
- AF is always irregularly irregular
- AF may co-exist with bundle branch block
- The possibility of Multi-focal atrial tachycardia and Atrial flutter should always be considered
Mis-treatment of Atrial Fibrillation:
- When a patient presents in new AF a secondary cause should be sort. Focusing on the AF alone in a patient with an acute underlying medical cause may be deleterious.
- Digoxin does not sufficiently limit AV nodal conduction when there is significant sympathetic discharge It should only be used as monotherapy in sedentary patients
- The greatest risk arising from AF is thromboembolic complications including stroke. Failure to assess and treat the stroke risk is a significant error.
- Patients with accessory pathways are at risk of sudden death if AF occurs. This risk is increased if the AV node is blocked. Therefore any patient with a known or suspected accessory pathway and an irregular rhythm should not be treated with any AV blocking agent including adenosine and B blockers
- Kirchhof P, Benussi S, Kotecha K et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J; 37: 38, 7
- NICE: Atrial Fibrillation: The management of atrial fibrillation.
- Lowenstein SR, Gabow PA, Cramer J, et al. The role of alcohol in new onset atrial fibrillation. Arch Intern Med 1983;143(10):1882-1885.
- Scheuermeyer FX. Emergency Department Patients With Atrial Fibrillation or Flutter and an Acute Underlying Medical Illness May Not Benefit From Attempts to Control Rate or Rhythm. Ann Emerg Med 2015; 65(5):511-522
- Recusitation council UK. Advances life support – Peri-arrest arrhythmias.
- Carlson J, Miketic S, Windeler J et al. randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) Study. J Am Coll Cardiol. 2003;41(10):1690-1696)
- Van Gelder IC, Groenveld HF, Crijns HJ, Tuininga YS, Tijssen JG, Alings AM, Hillege HL, Bergsma-Kadijk JA, Cornel JH, Kamp O, Tukkie R, Bosker HA, Van Veldhuisen DJ, Van den Berg MP, RACE II Investigators . Lenient versus strict rate control in patients with atrial fibrillation . N Engl J Med. 2010; 362: 1363 1373








48 Comments
Good quick reminder
Fantastic article – collates information normally found on several different articles or book chapters into ED one ED relevant document.
Quick recap of AF – useful resource
good review of AF, helpful in terms of management .
very concise and helpful for everyday use in the ED
Short and simple guide. Very useful
Good and quick review of a fib .
Very useful session and revision. Very common presentation and requires good knowledge all the time
excellent review
A brief very informative session
Very useful revision material
very informative and concise
really good article
Useful reminder, thank you
Helpful thank you
Nice short af management topic
very practical and informative esp in emergency setting Thanks
Good recap
Good flow diagram summary
great info
very useful
Comprehensive approach to AF. Refreshed the knowledge.
Very helpful session about AF.
great read, I enjoyed every bit
Very usefull comprehensive information.
nice and practical approach to AF in ED
Simple, precise and practically useful topic
Good
It was quite helpful and refreshing . The algorithm was helpful.
nice flowchart
Useful revision exercise
Written with how AF could be best managed in ED BY ED physicians. Excellent.
Excellent reviews
Great read.Very concise and practical for ED
Good short review of the practical management of AF in ED
a useful revision of an important topic. Thank you
useful
very useful article ,refresh your knowledge
Great module! Very practical, easy to follow and greatful to the ease of understanding!
very good module
Well presented, really helpful reference for ED physicians.
Great module; simple and practical
Very useful information
very useful
Excellent
Very handy review of AF
Very focused approach.
Thanks RECM for providing exact ED based management
Good to the point approach .well done